1,4-Dihydropyridines: discovery of minimal AIEEgens and their mitochondrial imaging applications†
Abstract
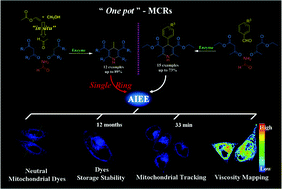
Typical aggregation-induced emission enhancement fluorogens (AIEEgens) generally are designed as propeller-shaped molecules with multiple aromatic rotors. Described herein are 1,4-dihydropyridines, which have the minimum size necessary for AIEE, containing only a single ring, synthesized through a facile biocatalysis procedure. Owing to the AIEE property, intramolecular motion can be restrained by the surrounding environment, causing the radiative channel to open up. Both the fluorescence intensities and the lifetimes of the 1,4-dihydropyridine representatives increased with increasing viscosity, and high sensitivities were observed. On the basis of the single crystal X-ray structures, density functional theory (DFT) calculations were performed to explain the mechanism of the single-ring AIEE behaviour. Moreover, a few of the neutral AIEEgens were found to possess a high specificity towards mitochondria. As an example, one of the AIEEgens exhibited superior photostability and excellent storage tolerance, allowing for real-time imaging, viscosity mapping, and long-term tracking of the dynamics of the mitochondrial morphology.
- This article is part of the themed collection: 2017 Journal of Materials Chemistry B HOT Papers

 Please wait while we load your content...
Please wait while we load your content...