The tunable effect of nitrogen and boron dopants on a single walled carbon nanotube support on the catalytic properties of a single gold atom catalyst: a first principles study of CO oxidation†
Abstract
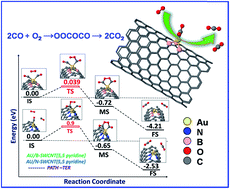
The mechanism of CO oxidation by O2 on a single Au atom supported on pristine and nitrogen or boron doped (5,5) and (8,0) single-walled carbon nanotubes (SWCNTs) has been systematically investigated theoretically using density functional theory. Fundamental aspects such as the optimized geometry, adsorption energy and charge transfer are elucidated to analyze the adsorption properties of CO and O2. The results reveal that boron and nitrogen doped SWCNTs can enhance the binding strength and catalytic activity of Au catalysts compared with the pristine SWCNTs. Moreover, the boron and nitrogen doping donate or withdraw charges to and from the Au atom. The excess positive or negative charges on Au can significantly modify its interactions with CO and O2 molecules. We have found that O2 binds stronger than CO on Au/B-SWCNTs, but weaker than CO on Au/SWCNTs and Au/N-SWCNTs. The adsorption preference of CO and O2 molecules on the doped SWCNTs gives a distinct coadsorption pattern of the reactants which is closely related to the dopant configurations. Furthermore, the calculations reveal that the reactions proceed via either the bi-molecular Langmuir–Hinshelwood (BLH) mechanism or the bi-molecular Eley–Rideal (BER) mechanism with a relatively low energy barrier of 0.22 and 0.28 eV. The peroxide-like (OOCO) complex is a key intermediate in the reaction pathway in both BER and BLH mechanisms. There are two different pathways from OOCO to the final product CO2: a two-step pathway, where two CO2 molecules are formed independently, and a self-promotion pathway, where the oxidation of the first CO molecule is promoted by the second CO molecule with a much lower barrier. Besides the traditional BLH and BER mechanisms, a tri-molecular Eley–Rideal (TER) mechanism is also observed, which is almost a barrierless process on Au/B-SWCNTs. In the tri-molecule mechanism, the oxygen molecule is activated by the pre-adsorbed CO molecule on the Au site which is not observed in the conventional LH and ER mechanisms. Our findings give a clear description of the relationship between the support and the catalytic properties of Au and demonstrate the possible ways to engineer the catalytic performance of Au catalysts by rational doping.
- This article is part of the themed collection: 2017 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...