Copper-substituted Na0.67Ni0.3−xCuxMn0.7O2 cathode materials for sodium-ion batteries with suppressed P2–O2 phase transition†
Abstract
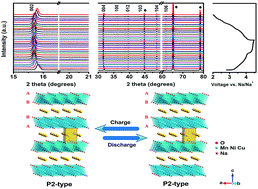
P2-type sodium layered oxides NaxMO2 (M = transition metal) are considered as one kind of promising cathode material for sodium-ion batteries because of their known structures, superior electrochemical properties, and their ease of synthesis. The Ni2+/Ni3+ and Ni3+/Ni4+ redox reactions endow the P2–Na2/3Ni1/3Mn2/3O2 electrode with a relatively high operating voltage and high specific capacity. However, the phase transition from P2 to O2 and Na+/vacancy ordering make P2–Na2/3Ni1/3Mn2/3O2 susceptible to severe voltage and capacity decay. Herein, we propose to employ the electrochemically active copper(II) as a unique substituent to stabilize the P2 phase, forming Na0.67Ni0.3−xCuxMn0.7O2 (x = 0, 0.1, 0.2 and 0.3). Our work highlights the importance of Cu(II) in the structural engineering of high performance cathode materials, whose existence can not only stabilize the P2 phase against the notorious phase transition, but also contribute to the rechargeable capacity due to the high potential Cu2+/Cu3+ redox. We identified that the cathode formulated as P2-type Na0.67Ni0.1Cu0.2Mn0.7O2 shows favorable battery performance with much-alleviated structural degradation.


 Please wait while we load your content...
Please wait while we load your content...