Molybdenum diboride nanoparticles as a highly efficient electrocatalyst for the hydrogen evolution reaction†
Abstract
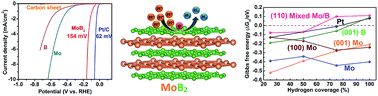
Non-noble metal nanomaterials (molybdenum sulfides, phosphides, carbides, and nitrides) have recently emerged as highly active electrocatalysts for the hydrogen evolution reaction (HER). Molybdenum borides in contrast have not been studied for their HER activity at the nanoscale, however, they were recently shown to be already efficient HER catalysts in the bulk (microscale). In this study, we report on the first nanocrystalline molybdenum boride (MoB2) synthesized by a simple, one-step, relatively low temperature (650 °C) and environmentally benign redox-assisted solid state metathesis (SSM) reaction. The obtained MoB2 nanospheres exhibit a low onset overpotential of 154 mV at 10 mA cm−2, a Tafel slope of 49 mV dec−1 and high stability. Furthermore, density functional theory (DFT) calculations show that several surfaces are active and that the optimum evolution of H2 occurs at a hydrogen coverage between 75% and 100% on the B-terminated {001} surface. These experimental and theoretical results open new avenues to design new architectures of inexpensive and highly efficient boron-based HER catalysts, such as boride nanospheres (with maximum active sites) or materials with B-terminated surfaces (e.g. {001} nanosheets of AlB2-type borides or even the recently discovered borophene and related 2D compounds).


 Please wait while we load your content...
Please wait while we load your content...