Methodology and applications of the hexadehydro-Diels–Alder (HDDA) reaction
Abstract
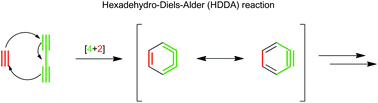
The hexadehydro-Diels–Alder (HDDA) reaction between an alkyne and a 1,3-diyne has recently become a rapidly growing area in the field of aryne chemistry. Both concerted and stepwise mechanisms for HDDA are energetically and geometrically feasible. The formation of a reactive benzyne intermediate under thermal conditions has been coupled with a wide variety of intra- and intermolecular trapping reactions to access highly functionalised aromatic compounds. With this in mind, reagents can be tailored to generate compounds for desired applications such as the synthesis of bioactive molecules or optoelectronic materials. This review presents a comprehensive overview of the HDDA reaction to date.
- This article is part of the themed collections: 2017 Organic Chemistry Frontiers Review-type Articles and Novel π-electron molecular scaffolds


 Please wait while we load your content...
Please wait while we load your content...