Bridge-driven aggregation control in dibenzofulvene–naphthalimide based donor–bridge–acceptor systems: enabling fluorescence enhancement, blue to red emission and solvatochromism†
Abstract
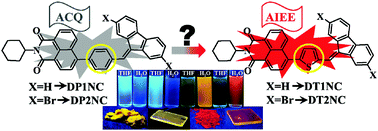
Four novel 1,8-naphthalimide (NC) and mono-substituted dibenzofulvene (DBF) based structures have been designed and successfully synthesized. Two luminogens were substituted by a thiophene bridge (DT1NC and DT2NC), while the other two luminogens were substituted by a phenyl bridge (DP1NC and DP2NC) between the NC and DBF units. This minor structural modification crafts striking changes in the photophysical behavior. DT1NC and DT2NC displayed aggregation induced emission enhancement (AIEE) behavior. They also showed orange and red emission (575 nm and 602 nm), respectively, with large bathochromic shifts (35 nm and 112 nm) and high quantum yields (84.10% and 65.65%) in the aggregated state because of ladder type J-aggregation. DP1NC exhibited weak AIEE behavior in the blue region, while DP2NC showed an AIE inactive nature because of the strong C–H⋯π intermolecular interactions. All the luminogens showed positive solvatochromism caused by intramolecular charge transfer (ICT). The DT2NC and DP2NC luminogens were substituted with two bromine atoms at the 2,7 positions of the DBF moiety, but unexpectedly only DT2NC showed a strong heavy atom effect. Theoretical and experimental HOMO and LUMO energy levels were estimated by density functional theory and cyclic voltammetry, respectively. All luminogens displayed excellent thermal stabilities and good morphological behavior.


 Please wait while we load your content...
Please wait while we load your content...