Mild and efficient synthesis of ω,ω-heterodifunctionalized polymers and polymer bioconjugates†
Abstract
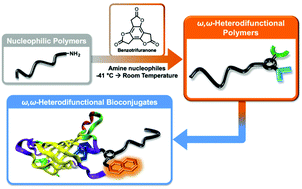
This report describes the efficient preparation of ω,ω-heterodifunctionalized polymers and polymer bioconjugates under mild conditions using a recently introduced reagent, benzotrifuranone (BTF). Demonstrated is how BTF enables introduction of differentially “clickable” functional groups (e.g., alkenes and alkynes) to monomethyl ether poly(ethylene glycol) amine at ambient temperature using near-stoichiometric amounts of reagents, in contrast to conventional polymer heterofunctionalization approaches that may require high temperatures, significant excesses of reagents, and/or numerous synthetic steps. Showcasing the methodology is facile access to an ω,ω-heterodifunctional polymer bearing a fluorescent (coumarin) dye and biotin. Found, in addition to avidin binding by the polymer bioconjugate, is the unexpected disruption of avidin tetramer formation.


 Please wait while we load your content...
Please wait while we load your content...