Perylenediimide-based glycoclusters as high affinity ligands of bacterial lectins: synthesis, binding studies and anti-adhesive properties†
Abstract
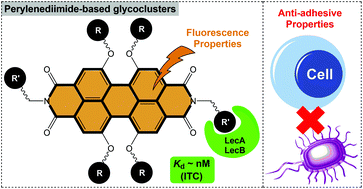
The synthesis of eight perylenediimide-based glycoclusters was readily performed from hexa- and tetra-propargylated cores through azide–alkyne “click” conjugation. Variations in the carbohydrate epitope (Glc, Gal, Man, Fuc) and the linker arm provided molecular diversity. Interactions with LecA and LecB, two proteins involved in the adhesion of Pseudomonas aeruginosa to host tissues, were evaluated by microcalorimetry (ITC). In both cases high affinities were obtained with Kd values in the nanomolar range. Further evaluation of their anti-adhesive properties using cultured epithelial cells demonstrated their potent anti-adhesive activities against Pseudomonas aeruginosa with only 30–40% residual adhesion observed. The fluorescence properties of the PDI core were then investigated by confocal microscopy on cell–bacteria cultures. However, the red fluorescence signal of the PDI-based glycocluster was too weak to provide significant data. The present study provides another type of anti-adhesive glycocluster against bacterial infection with a large aromatic PDI core.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...