Switching substitution groups on the in-tether chiral centre influences backbone peptides’ permeability and target binding affinity†
Abstract
Different substitution groups on the in-tether chiral centre of chirality-induced helical peptides (CIH peptides) showed distinguishable effects on the peptides’ cellular uptakes and binding affinities with the estrogen receptor α(ER-α). This study proves that in-tether chiral centres are a valuable modification site for constructing peptide ligands with preferable biophysical properties.
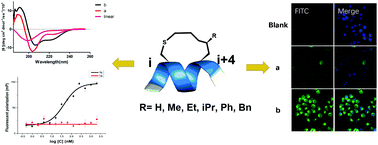

 Please wait while we load your content...
Please wait while we load your content...