Enabling syntheses of diterpenoid alkaloids and related diterpenes by an oxidative dearomatization/Diels–Alder cycloaddition strategy
Abstract
Covering: 2015 to March 2017
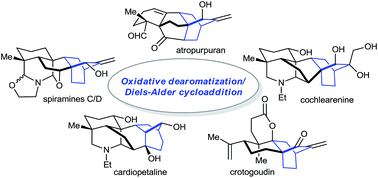
ortho-Benzoquinones generated from dearomatization of aromatic compounds are highly reactive intermediates in organic synthesis. One of their most important applications involves the participation in Diels–Alder cycloadditions, facilitating rapid access to molecular complexity. This powerful strategy has recently been utilized in the syntheses of diverse diterpenoid alkaloids and their biogenetically relevant diterpenes, which is the subject of this Highlight.


 Please wait while we load your content...
Please wait while we load your content...