Designing the thermal behaviour of aqueous biphasic systems composed of ammonium-based zwitterions†
Abstract
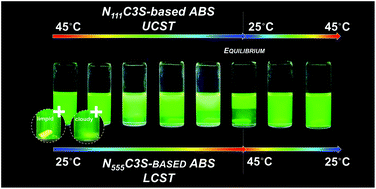
The ability of water-soluble ammonium-based zwitterions (ZIs) to form aqueous biphasic systems (ABS) in the presence of aqueous solutions of salts is disclosed here. These systems are thermoreversible at temperatures close to room temperature and further allow the design of their thermal behavior, from an upper critical solution temperature (UCST) to a lower critical solution temperature (LCST), by increasing the ZI alkyl chain length. The investigated thermoreversible ABS are more versatile than typical liquid–liquid systems, and can be applied in a wide range of temperatures and compositions envisaging a target separation process. An example is given regarding their ability to selectively separate mixtures of aromatic and aliphatic amino acids in a single-step.
- This article is part of the themed collections: International Symposium on Green Chemistry 2017 and 2017 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...