High pressure water-initiated ring opening polymerization for the synthesis of well-defined α-hydroxy-ω-(carboxylic acid) polycaprolactones
Abstract
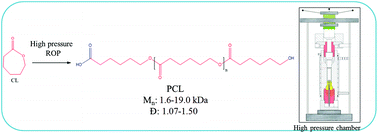
The development of a green ε-caprolactone (CL) polymerization method is the most important challenge in modern polymer chemistry. Herein, we propose a novel method to produce polyesters of a well-defined chemical structure, and narrow molecular weight distributions by using a combination of high pressure, temperature and water. We found two competitive phenomena: polymerization and crystallization occurring at high compression. Once the latter process is avoided, the control over the reaction is reached. By varying the thermodynamic conditions and concentration of water molecules, polymers of moderate molecular weights (Mn = 1.6–19.0 kg mol−1) and dispersities (Đ ≈ 1.07–1.50) could be produced. Additional NMR experiments supported by further MALDI TOF analysis enabled us to determine telechelic hydroxyl and carboxylic/acidic moieties as terminal groups of produced PCLs. Finally, the latter measurements also indicated that generally an increase in dispersity above 1.3 is strongly related to the competitive reactions leading to the production of cyclic polyesters. The presented results open up alternative routes to produce PCLs of enhanced biocompatibility and biodegradability using the greenest method reported to date.


 Please wait while we load your content...
Please wait while we load your content...