Formation of bioactive transformation products during glucocorticoid chlorination†
Abstract
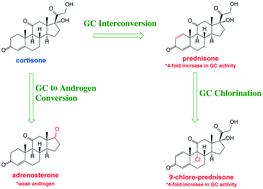
Glucocorticoid (GC) release into the environment has led to widespread detection of glucocorticoid receptor (GR) activity in water resources that has been shown to persist throughout conventional and some advanced wastewater treatment processes. Here, we used high performance liquid chromatography, high resolution mass spectrometry and nuclear magnetic resonance spectroscopy to explore the reaction of natural (cortisone, cortisol) and synthetic (prednisone, prednisolone, dexamethasone) GCs with free chlorine (HOCl) to simulate their fate during chemical disinfection of water and wastewater. Generally, GCs react slowly (t1/2 ∼ 7–200 h) with HOCl when compared to other steroid classes, but they yield complex mixtures of transformation products, with at times the majority of product mass comprising structurally identifiable and likely bioactive steroids. For example, we frequently observed chlorination at the C-9 position (e.g., 9-chloro-prednisone), a reaction known to increase GC activity 4-fold. We also identified reaction products in the adrenosterone family of androgens produced via cleavage of the C-17 side-chain on many GCs. Another common transformation pathway was the conversion of endogenous GCs to their more potent synthetic analogs via oxidation at the C-1/C-2 positions, with unsaturation reported to increase GR activity 4-fold (e.g., cortisol to prednisolone). Despite identification of such products, in vitro assays generally suggest GR activity decreases with extent of parent decay during chlorination. Cortisol was the exception, with GR activity only decreasing 2-fold in product mixtures (based on measured EC50 values) despite a 95% reduction in parent concentration, a result attributable to formation of the more potent prednisolone during chlorination. Furthermore, our assay likely underestimates product bioactivity as it did not account for the activity of several identified GC byproducts that first require in vivo activation via C-11 reduction, nor did it consider androgen receptor (AR) activity associated with byproducts from the adrenosterone family. To avoid formation of product mixtures with conserved bioactivity, advanced chemical oxidation processes may represent a more promising approach; we show that GCs react much more rapidly with ozone (t1/2 ∼ 0.4–1.3 min) and produce no observable UV-active products. This suggests disruption of the GC conjugated π-electron and ring systems, thereby likely mitigating biological activity.


 Please wait while we load your content...
Please wait while we load your content...