Predicting the phospholipophilicity of monoprotic positively charged amines†
Abstract
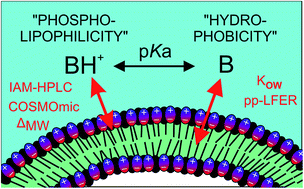
The sorption affinity of eighty-six charged amine structures to phospholipid monolayers (log KIAM) was determined using immobilized artificial membrane high-performance liquid chromatography (IAM-HPLC). The amine compounds covered the most prevalent types of polar groups, widely ranged in structural complexity, and included forty-seven pharmaceuticals, as well as several narcotics and pesticides. Amine type specific corrective increments were used to align log KIAM data with bilayer membrane sorption coefficients (KMW(IAM)). Using predicted sorption affinities of neutral amines, we evaluated the difference (scaling factor ΔMW) with the measured log KMW(IAM) for cationic amines. The ΔMW values were highly variable, ranging from −2.37 to +2.3 log units. For each amine type, polar amines showed lower ΔMW values than hydrocarbon based amines (CxHyN+). COSMOmic software was used to directly calculate the partitioning coefficient of ionic structures into a phospholipid bilayer (KDMPC–W,cation), including quaternary ammonium compounds. The resulting root mean square error (RMSE) between log KDMPC–W,cation and log KMW(IAM) was 0.83 for all eighty-six polar amines, and 0.47 for sixty-eight CxHyN+ amines. The polar amines were then split into five groups depending on polarity and structural complexity, and corrective increments for each group were defined to improve COSMOmic predictions. Excluding only the group with sixteen complex amine structures (≥4 polar groups, Mw > 400, including several macrolide antibiotics), the resulting RMSE for corrected KDMPC–W,cation values improved to 0.45 log units for the remaining set of 138 polar and CxHyN+ amines.
- This article is part of the themed collection: QSARs and computational chemistry methods in environmental chemical sciences


 Please wait while we load your content...
Please wait while we load your content...