Toward inert paramagnetic Ni(ii)-based chemical exchange saturation transfer MRI agents†
Abstract
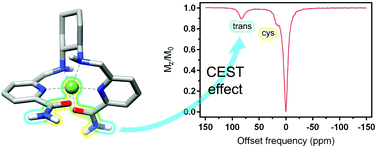
The Ni2+ complexes with hexadentate ligands containing two 6-methylpicolinamide groups linked by ethane-1,2-diamine (dedpam) or cyclohexane-1,2-diamine (chxdedpam) spacers were investigated as potential contrast agents in magnetic resonance imaging (MRI). The properties of the complexes were compared to that of the analogues containing 6-methylpicolinate units (dedpa2− and chxdedpa2−). The X-ray structure of the [Ni(dedpam)]2+ complex reveals a six-coordinated metal ion with a distorted octahedral environment. The protonation constants of the dedpa2− and dedpam ligands and the stability constants of their Ni2+ complexes were determined using pH-potentiometry and spectrophotometric titrations (25 °C, 0.15 M NaCl). The [Ni(dedpa)] complex (log KNiL = 20.88(1)) was found to be considerably more stable than the corresponding amide derivative [Ni(dedpam)]2+ (log KNiL = 14.29(2)). However, the amide derivative [Ni(chxdedpam)]2+ was found to be considerably more inert with respect to proton-assisted dissociation than the carboxylate derivative [Ni(chxdedpa)]. A detailed 1H NMR and DFT study was conducted to assign the 1H NMR spectra of the [Ni(chxdedpa)] and [Ni(chxdedpam)]2+ complexes. The observed 1H NMR paramagnetic shifts were found to be dominated by the Fermi contact contribution. The amide resonances of [Ni(chxdedpam)]2+ at 91.5 and 22.2 ppm were found to provide a sizeable chemical exchange saturation transfer effect, paving the way for the development of NiCEST agents based on these rigid non-macrocyclic platforms.


 Please wait while we load your content...
Please wait while we load your content...