An efficient Cu-catalyzed azide–alkyne cycloaddition (CuAAC) reaction in aqueous medium with a zwitterionic ligand, betaine†
Abstract
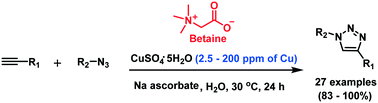
Cu-catalyzed azide–alkyne cycloaddition reaction in aqueous medium was dramatically accelerated by a simple zwitterionic additive, betaine, at ambient temperature. In the presence of betaine, 1,4-disubstituted-1,2,3-triazoles were obtained from azides and terminal alkynes in excellent yields under 2.5–200 ppm levels of Cu(I) in water. This method resulted in the remarkable reduction of the Cu(I) concentration down to ppm levels. The advantages of this protocol include the use of a conventional Cu catalytic system with commercially available betaine, which is generally non-toxic for biological application. Therefore, this efficient catalytic system should have broad applications in life science research and materials science.
- This article is part of the themed collection: 2017 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...