Fe atoms trapped on graphene as a potential efficient catalyst for room-temperature complete oxidation of formaldehyde: a first-principles investigation†
Abstract
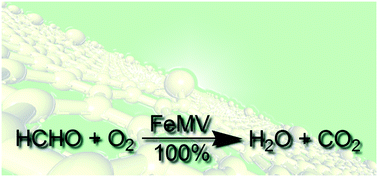
We investigated the oxidation of formaldehyde, one of the major indoor air pollutants, into CO2 and H2O over Fe atoms trapped in defects on graphene by first-principles based calculations. These trapped Fe atoms are not only stable to withstand interference from the reaction environments but are also efficient in catalyzing the reactions between coadsorbed O2 and formaldehyde. The oxidation of formaldehyde starts with the formation of a peroxide-like intermediate and continues by its dissociation into η1-OCHO coadsorbed with an OH radical. Then, the adsorbed OCHO undergoes conformational changes and hydride transfer, leading to the formation of H2O and CO2. Subsequent adsorption of O2 or formaldehyde facilitates desorption of H2O and a new reaction cycle initiates. The calculated barriers for formation and dissociation of the peroxide-like intermediate are 0.43 and 0.40 eV, respectively, and those for conformation changes and hydride transfer are 0.47 and 0.13 eV, respectively. These relatively low barriers along the reaction path suggest the potential high catalytic performance of trapped Fe atoms for formaldehyde oxidation.


 Please wait while we load your content...
Please wait while we load your content...