Proton conduction in alkali metal ion-exchanged porous ionic crystals†
Abstract
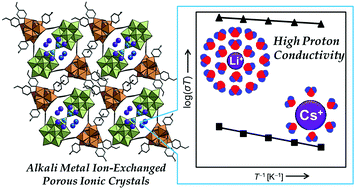
Proton conduction in alkali metal ion-exchanged porous ionic crystals A2[Cr3O(OOCH)6(etpy)3]2[α-SiW12O40]·nH2O [I-A+] (A = Li, Na, K, Cs, etpy = 4-ethylpyridine) is investigated. Single crystal and powder X-ray diffraction measurements show that I-A+ possesses analogous one-dimensional channels where alkali metal ions (A+) and water of crystallization exist. Impedance spectroscopy and water diffusion measurements of I-A+ show that proton conductivities are low (10−7–10−6 S cm−1) under low relative humidity (RH), and protons mostly migrate as H3O+ with H2O as vehicles (vehicle mechanism). The proton conductivity of I-A+ increases with the increase in RH and is largely dependent on the types of alkali metal ions. I-Li+ shows a high proton conductivity of 1.9 × 10−3 S cm−1 (323 K) and a low activation energy of 0.23 eV under RH 95%. Under high RH, alkali metal ions with high ionic potentials (e.g., Li+) form a dense and extensive hydrogen-bonding network of water molecules with mobile protons at the periphery, which leads to high proton conductivities and low activation energies via rearrangement of the hydrogen-bonding network (Grotthuss mechanism).


 Please wait while we load your content...
Please wait while we load your content...