Indirect dynamics in SN2@N: insight into the influence of central atoms†
Abstract
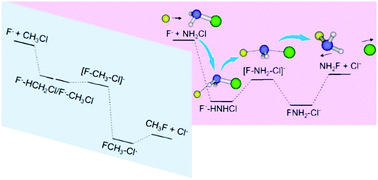
Central atoms have a significant influence on the reaction kinetics and dynamics of nucleophilic substitution (SN2). Herein, atomistic dynamics of a prototype SN2@N reaction F− + NH2Cl is uncovered employing direct dynamics simulations that show strikingly distinct features from those determined for a SN2@C congener F− + CH3Cl. Indirect scattering is found to prevail, which proceeds predominantly through a hydrogen-bonded F−–HNHCl complex in the reactant entrance channel. This unexpected finding of a pronounced contribution of indirect reaction dynamics, even at a high collision energy, is in strong contrast to a general evolution from indirect to direct dynamics with enhanced energy that characterizes SN2@C. This result suggests that the relative importance of different atomic-level mechanisms may depend essentially on the interaction potential of reactive encounters and the coupling between inter- and intramolecular modes of the pre-reaction complex. For F− + NH2Cl the proton transfer pathway is less competitive than SN2. A remarkable finding is that the more favorable energetics for NH2Cl proton transfer, as compared to that for CH3Cl, does not manifest itself in the reaction dynamics. The present work sheds light on the underlying reaction dynamics of SN2@N, which remain largely unclear compared to well-studied SN2@C.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...