Excess charge driven dissociative hydrogen adsorption on Ti2O4−†
Abstract
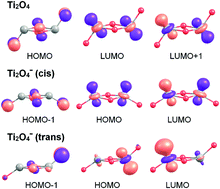
The mechanism of dissociative D2 adsorption on Ti2O4−, which serves as a model for an oxygen vacancy on a titania surface, is studied using infrared photodissociation spectroscopy in combination with density functional theory calculations and a recently developed single-component artificial force induced reaction method. Ti2O4− readily reacts with D2 under multiple collision conditions in a gas-filled ion trap held at 16 K forming a global minimum-energy structure (DO–Ti–(O)2–Ti(D)–O)−. The highly exergonic reaction proceeds quasi barrier-free via several intermediate species, involving heterolytic D2-bond cleavage followed by D-atom migration. We show that, compared to neutral Ti2O4, the excess negative charge in Ti2O4− is responsible for the substantial lowering of the D2 dissociation barrier, but does not affect the molecular D2 adsorption energy in the initial physisorption step.


 Please wait while we load your content...
Please wait while we load your content...