Sodium–carboxylate contact ion pair formation induces stabilization of palmitic acid monolayers at high pH†
Abstract
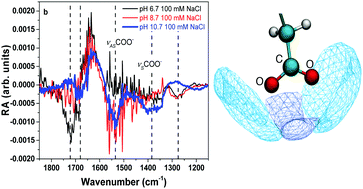
Sea spray aerosols (SSA) are known to have an organic coating that is mainly composed of fatty acids. In this study, the effect of pH and salt on the stability and organization of a palmitic acid (PA) monolayer is investigated by surface vibrational spectroscopy and molecular dynamics simulations. Results indicate that alkyl chain packing becomes more disordered as the carboxylic headgroup becomes deprotonated. This is associated with packing mismatch of charged and neutral species as charged headgroups penetrate deeper into the solution phase. At pH 10.7, when the monolayer is ∼99% deprotonated, palmitate (PA−) molecules desorb and solubilize into the bulk solution where there is spectroscopic evidence for aggregate formation. Yet, addition of 100 mM NaCl to the bulk solution is found to drive PA− molecules to the aqueous surface. Free energy calculations show that PA− molecules become stabilized within the interface with increasing NaCl concentration. Formation of contact –COO−:Na+ pairs alters the hydration state of PA− headgroups, thus increasing the surface propensity. As salts are highly concentrated in SSA, these results suggest that deprotonated fatty acids may be found at the air–aqueous interface of aerosol particles due to sea salt's role in surface stabilization.


 Please wait while we load your content...
Please wait while we load your content...