Synthesis and photophysical properties of new through-space conjugated luminogens constructed by folded tetraphenylethene†
Abstract
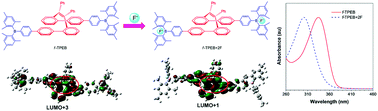
Through-space conjugation refers to a unique non-covalent electronic coupling interaction typically occurring between two face-to-face parallel aromatic units, which has shown great potential in constructing novel functional materials capable of multidimensional carrier and energy transportation. However, well-studied through-space conjugation systems are quite rare. In this work, a series of tailored through-space conjugated luminogens are readily constructed from the folded tetraphenylethene (TPE) core and common functional groups like fluorene, carbazole, imidazole and dimesitylborane. Systematic studies on their photophysical properties were conducted, and a deep insight into the structure–property relationship is gained. The new molecules show aggregation-enhanced emission (AEE) characteristics with high fluorescence quantum efficiencies of up to 54% in films. Through the binding experiments of fluoride ions with boron atoms, the impacts of through-space conjugation on the photophysical properties of the luminogens are carefully studied. All these results undoubtedly provide important clues to the rational design of efficient through-space conjugated materials with specific functions.


 Please wait while we load your content...
Please wait while we load your content...