Highly efficient orange-red electroluminescence of iridium complexes with good electron mobility†
Abstract
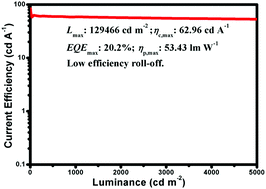
Two iridium complexes with 1-(2,6-bis(trifluoromethyl)pyridin-4-yl)isoquinoline (tfmpiq) and 4-(2,6-bis(trifluoromethyl)pyridin-4-yl)quinazoline (tfmpqz) main ligands and a tetraphenylimidodiphosphinate (tpip) ancillary ligand were applied in organic light-emitting diodes (OLEDs). The introduction of quinazoline greatly influences the nature of the complex. The quantum yield and the electron mobility of Ir(tfmpqz)2(tpip) are much higher than those of Ir(tfmpiq)2(tpip) (Ir(tfmpiq)2(tpip): Φ: 0.47, μe: 8.93–9.47 × 10−6 cm2 V−1 s−1 under an electric field from 1040 (V cm−1)1/2 to 1300 (V cm−1)1/2; Ir(tfmpqz)2(tpip): Φ: 0.98, μe: 6.44–7.20 × 10−6 cm2 V−1 s−1 under an electric field from 1040 (V cm−1)1/2 to 1300 (V cm−1)1/2). In addition, the Ir(tfmpqz)2(tpip)-based device also displayed better performance than that using Ir(tfmpiq)2(tpip). Furthermore, with a europium complex, Eu(DBM)3phen (DBM = dibenzoylmethide; phen = 1,10-phenanthroline) as a sensitizer, the device based on Ir(tfmpqz)2(tpip) with a double emissive layer structure of ITO/MoO3 (3 nm)/TAPC (50 nm)/Ir(tfmpqz)2(tpip) (5 wt%):TcTa (10 nm)/Eu(DBM)3phen (0.2 wt%):Ir(tfmpqz)2(tpip) (5 wt%):26DCzPPy (10 nm)/TmPyPB (50 nm)/LiF (1 nm)/Al (100 nm) displayed the best performance with a maximum luminance of 129 466 cd m−2, and a maximum current efficiency and a maximum power efficiency of 62.96 cd A−1 and 53.43 lm W−1, respectively, with low efficiency roll-off. The current efficiency still remains as high as 58.84 cd A−1 at a brightness of 1000 cd m−2 and 53.27 cd A−1 at a brightness of 5000 cd m−2. These results suggest that Ir(III) complexes with quinazoline units are potential orange-red phosphorescent materials for OLEDs.


 Please wait while we load your content...
Please wait while we load your content...