New cyclopentadithiophene (CDT) linked porphyrin donors with different end-capping acceptors for efficient small molecule organic solar cells†
Abstract
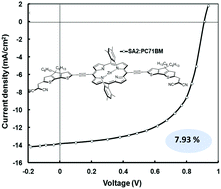
The synthesis of two new A–π–D–π–A molecules bearing a Zn-porphyrin core donor linked through cyclopenta[2,1-b:3,4-b′]dithiophene bridges to the electron-acceptor rhodanine (SA1) or dicyanovinylene (SA2) groups is described. The optical and electrochemical properties of these compounds are investigated. The horizontal conjugation of cyclopentadithiophene between the porphyrin core and the end-capping acceptor not only effectively increases the light harvesting between the Soret and Q bands of the porphyrin unit, but also optimizes molecular packing through linear π-conjugated backbones. Compounds SA1 and SA2 are employed as donors along with [6,6]-phenyl-C71-butyric acid methyl ester (PC71BM) as an acceptor in organic bulk heterojunction organic solar cells. After optimizing the processing of the active layer (a solvent additive and subsequent solvent vapor annealing), overall power conversion efficiencies of 6.71% and 7.93% (with low energy loss of 0.55 eV) are obtained for organic solar cells. The use of a solvent additive and subsequent solvent vapor annealing provide enhanced nanoscale morphology and bi-continuous interpenetrating networks in the active layer required for efficient exciton dissociation into free charge carriers and their subsequent transportation towards electrodes. This change led to higher PCE values when compared to devices based on as-cast active layers.


 Please wait while we load your content...
Please wait while we load your content...