Solid state emissive organic fluorophores with remarkable broad color tunability based on aryl-substituted buta-1,3-diene as the central core†
Abstract
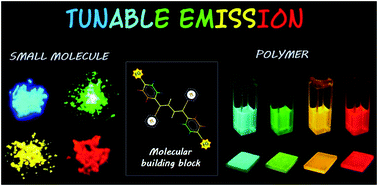
Multicolor emissive organic solid fluorophores are scarcely observed because of molecular aggregation in the condensed phase. However, rational molecular design is crucial to develop solid state emissive fluorophores from a simple core skeleton. Herein, the development of a series of small molecular derivatives and a conjugated copolymer having a (1Z,3Z)-1,4-diphenyl-1,3-butadiene unit as the central core has been reported. Notably, all fluorophores are highly emissive in the solid state and the emission colors cover the whole visible region from blue to red. Colour tuning has been simply achieved by varying the substituents attached with the aryl group of the central core. In addition, a partially twisted structure of the central core helps to reduce the intramolecular interaction and improve the photophysical properties of the fluorophores. Furthermore, electrochemical measurements indicate that polymer based fluorophores are potential candidates for organic photovoltaic (OPV) applications.


 Please wait while we load your content...
Please wait while we load your content...