Self-bonding and the electrochemical properties of silica-coated nanowires composed of cobalt-coordinated peptide bundles†
Abstract
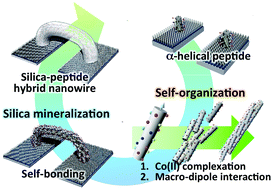
We propose a method for self-bonding between electrodes using the self-organizational processes of a silica-coated peptide hybrid nanowire. We designed and synthesized a 23-mer α-helical peptide having functional sites that serve as catalytic sites for silica mineralization (glutamic acid [Glu] and lysine [Lys]) and binding (serine [Ser]), as well as for the formation of a Co(II) ion complex. The peptides formed nanowires composed of α-helical bundles that showed axial connection because of complexation between Co(II) and the histidine (His) residues, and macro-dipole interactions of the α-helical peptides. The nanowires self-bonded between the electrodes, and the surface of the nanowires was coated with silica using mineralization. The silica coating of the surface of the Co(II)-coordinated peptide nanowires induced two kinds of phenomena: (1) structural stabilization of the peptide nanowire component in the composite and (2) an increase in conductivity compared with that of the non-coated peptide nanowires.


 Please wait while we load your content...
Please wait while we load your content...