Large-pore, silica particles with antibody-like, biorecognition sites for efficient protein separation†
Abstract
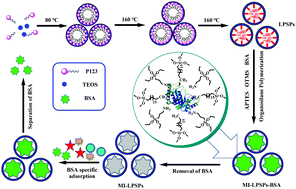
Natural antibodies are used widely for various applications such as in biomedical analysis, protein separation, and targeted-drug delivery, but they suffer from high cost and low stability. In this study, we developed a facile approach for the construction of antibody-like binding sites in a porous silica solid for efficient separation of bovine serum albumin (BSA) based on large-pore silica particles (LPSPs). This was accomplished by grafting two types of organosilane monomers, 3-aminopropyltriethoxylsilane (APTES) and octyltrimethoxysilane (OTMS), to provide hydrogen bonds or hydrophobic interactions with BSA through molecular imprinting technology. The resulting molecularly imprinted, large-pore silica particles (MI-LPSPs) were characterized by scanning electron microscopy (SEM), Fourier transform infrared (FT-IR) spectroscopy, X-ray photoelectron spectroscopy (XPS), thermogravimetric analysis (TG), X-ray diffraction (XRD) and N2 sorption analysis. Results showed that the as-synthesized MI-LPSPs exhibited a spherical morphology, favorable stability and large pore structure. The kinetic adsorption experiments showed that the MI-LPSPs could reach equilibrium within one hour and were described well by the pseudo second-order model, indicating that chemical adsorption might be the rate-limiting step. Meanwhile, the MI-LPSPs had a large binding capacity up to 162.82 mg g−1 and high selectivity for the recognition of BSA. Moreover, such a high binding capacity and selectivity was retained after six runs, indicating a good stability and reusability of MI-LPSPs. Thus, it is expected that a simple synthetic methodology in the present study provides a promising pathway to prepare novel imprinted materials for efficient purification and separation of target proteins.


 Please wait while we load your content...
Please wait while we load your content...