Improving endothelialization by the combined application of polyethylene glycol coated cerium oxide nanoparticles and VEGF in electrospun polyurethane scaffolds†
Abstract
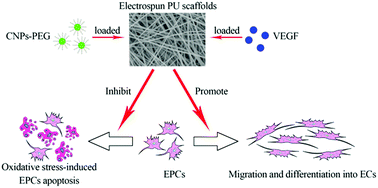
Synthetic small-diameter vascular grafts are of limited use mainly due to the lack of endothelial cells (ECs), which inhibit intraluminal thrombosis and intimal hyperplasia. Grafts loaded with homing factors for circulating endothelial progenitor cells (EPCs) have the potential to achieve in situ endothelialization. In view of the important role that EPCs play in the construction of small-diameter artificial blood vessels, antioxidant therapy aiming to inhibit oxidative stress-induced EPC apoptosis should be the focus of clinical interest. In this study, polyethylene glycol coated cerium oxide nanoparticles (CNPs-PEG) with antioxidant properties were successfully synthesized and characterized. The effects of CNPs-PEG on EPC viability and EPC apoptosis induced by oxidative stress were examined. Then, CNPs-PEG together with a potent angiogenesis cytokine vascular endothelial growth factor (VEGF) were encapsulated into polyurethane (PU) scaffolds via electrospinning. The morphology, mechanical properties and CNPs-PEG/VEGF release profiles of the scaffolds were investigated. The growth of EPCs on the scaffolds, and the effects of the released CNPs-PEG and VEGF on anti-EPC apoptosis and endothelialization in vitro were studied. The results showed that CNPs-PEG had favorable stability and cytocompatibility. They could effectively inhibit H2O2-induced EPC apoptosis. The scaffolds showed sustained release behavior of CNPs-PEG/VEGF and favorable cytocompatibility. The released CNPs-PEG retained the anti-apoptosis properties and, moreover, enhanced the effects of VEGF on the mobilization and differentiation of EPCs. It is concluded that the combined application of CNPs-PEG and VEGF in electrospun PU scaffolds facilitated endothelialization in vitro, and thus should be promising for the construction of small-diameter artificial blood vessels.


 Please wait while we load your content...
Please wait while we load your content...