Phenyl and thienyl functionalized imidazolium iodides for highly efficient quasi-solid-state dye-sensitized solar cells†
Abstract
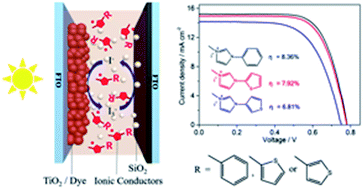
To enhance the ionic conductivity of imidazolium iodide based ionic conductors, the π conjugation of the imidazolium cation is expanded via N-substitution with aromatic groups of phenyl and thienyl. Three ionic conductors (ICs) of MPhII, MT2II and MT3II are designed and synthesized by linking benzene, 2-thiophene, and 3-thiophene to the imidazolium ring, respectively, and their single crystal structures are revealed. Quasi-solid-state electrolytes are prepared by solidifying the IC-containing solutions with SiO2 nanoparticles for quasi-solid-state dye-sensitized solar cells (QSS-DSSCs). The ionic conductivity of these quasi-solid-state electrolytes depends on the substituent and the substitution position as well, which correlates well with the distance between adjacent iodides, as revealed by the crystal packing structures. Owing to the highest conductivity among the three electrolytes, the QSS-DSSC with the MPhII based quasi-solid-state electrolyte achieves the highest power conversion efficiency of 8.36% under AM 1.5G sunlight (100 mW cm−2), which also exhibits good long-term stability under one sun soaking for more than 2000 h.


 Please wait while we load your content...
Please wait while we load your content...