Enhanced Li+ conduction in perovskite Li3xLa2/3−x□1/3−2xTiO3 solid-electrolytes via microstructural engineering†
Abstract
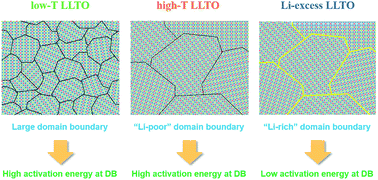
Solid electrolytes are key to the evolution of all-solid-state lithium batteries as next-generation energy storage systems. High ionic conductivity and stability of solid electrolytes are critical requirements for designing reliable all-solid-state lithium batteries. Perovskite-type lithium lanthanum titanates Li3xLa(2/3)−x□(1/3)−2xTiO3 (LLTOs) have received much attention as a potential inorganic solid electrolyte to replace current organic liquid electrolytes; however, the practical use of LLTOs is limited by their low total conductivity. With the aim of improving the ionic conductivity, we investigated a correlation between the microstructures and Li+ conducting properties of LLTO perovskites. We show that the total conductivity of LLTOs is dominated by the domain boundary resistance, and the synthesis condition of the electrolyte (sintering temperature and Li concentration) has a crucial role in modifying the microstructure and composition of the domain boundaries to significantly reduce the boundary resistance. By controlling the sintering temperature and Li content, in particular, a total Li+ conductivity as high as 4.8 × 10−4 S cm−1 can be achieved at room temperature. The findings of this study would be essential in understanding Li+ conducting behaviors and in developing highly conductive perovskite-type LLTO solid electrolytes.


 Please wait while we load your content...
Please wait while we load your content...