Artificial photosynthesis of methanol from carbon dioxide and water via a Nile red-embedded TiO2 photocathode†
Abstract
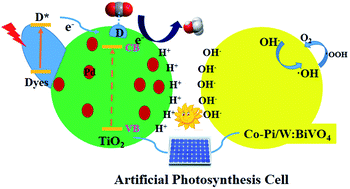
The conversion of carbon dioxide into useful chemicals is a prospective strategy for alleviating the greenhouse effect and the depletion of energy. Herein, we report an artificial photosynthetic system composed of a photoanode and a photocathode comprised of NRx@TiO2 functionalized with Nile red via covalent linkage or Pd/NRx@TiO2 with additional palladium nanoparticles. The new Nile red derivatives and organic–inorganic composite electrodes were steadily prepared and well characterized using NMR, HRMS, UV-vis, FTIR, TEM, XPS, XRD and SEM. Methanol and oxygen were the products that could be detected in the liquid and gas phase. The main active species in this artificial photosynthesis system were proven using EPR spectroscopy to be hydroxy radicals releasing O2 gas via H2O2. Moreover, the carbon source of methanol was validated using a 13CO2 labeling experiment; 18O2 was determined to come from H2O using GC-MS. The optimal photoelectrocatalytic CO2 reduction was carried out using Pd/NR2@TiO2 as the working electrode yielding methanol at a rate of 106 μM h−1 cm−2 with high light quantum efficiency (Φcell = 0.95).


 Please wait while we load your content...
Please wait while we load your content...