Surfactant widens the electrochemical window of an aqueous electrolyte for better rechargeable aqueous sodium/zinc battery†
Abstract
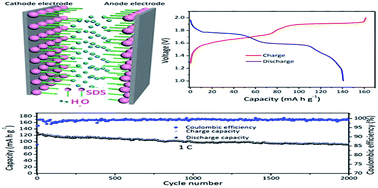
Aqueous rechargeable batteries have received significant attention because of their low-cost and security. However, the narrow electrochemical stability window (about 1.23 V) of the aqueous electrolyte sets a limit on their energy output. Herein, we have developed an aqueous rechargeable hybrid battery using Na2MnFe(CN)6 nanocubes as the cathode and a zinc metal sheet as the anode, which delivered a high energy density of 170 W h kg−1 and a capacity retention of 75% over 2000 cycles with an operating voltage of up to 2.0 V. By adding sodium dodecyl sulfate (SDS) to the aqueous electrolyte, the electrochemical stability window of the electrolyte was expanded to about 2.5 V. The results of the experiments and calculations based on the density functional theory indicate that SDS can not only inhibit the decomposition of water, suppress the dissolution of Mn and the corrosion of zinc but also increase the cycle life and rate capability. The low-cost, high energy density, and long cycle life of the battery suggest that it is a promising candidate for energy storage applications.

 Please wait while we load your content...
Please wait while we load your content...