In situ polymerization induced supramolecular hydrogels of chitosan and poly(acrylic acid-acrylamide) with high toughness†
Abstract
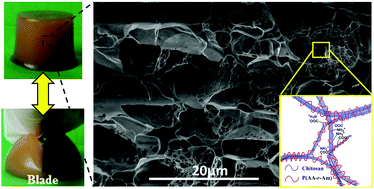
A novel type of supramolecular hydrogel was developed by in situ polymerization of acrylic acid (AA) and acrylamide (AM) monomers in the aqueous solution of chitosan (CS), in which nanofiber-structured CS and polyelectrolyte chains of poly(acrylic acid-acrylamide) P(AA-r-AM) form the dynamic cross-linked network through the electrostatic interaction of ions. 1H NMR, WAXD, SEM and TEM were used to trace the formation of this supramolecular hydrogel, and revealed that CS chains aggregate into a nanofiber when the copolymerization of AA and AM proceeded. The obtained hydrogels exhibited good comprehensive mechanical properties. With the excellent fatigue resistance and a high toughness of ∼500 J m−3, the tensile strength and elongation of hydrogel-5 are 120 kPa and 1600%, respectively. The obtained supramolecular hydrogel can self-heal and exhibited no residual strain after continuous deformation-resting processes and loading–unloading tests. Furthermore, the obtained hydrogels can be used to adsorb metal ions in water, interestingly their tensile modulus enhanced several times after adsorption of metal ions.

 Please wait while we load your content...
Please wait while we load your content...