Thermo and pH dual responsive polypeptides derived from “clickable” poly(γ-3-methylthiopropyl-l-glutamate)†
Abstract
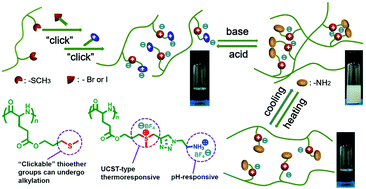
Poly(γ-3-methylthiopropyl-L-glutamate) (PMTPLG) with “clickable” thioether groups can be readily prepared from n-butylamine initiated ring-opening polymerization of γ-3-methylthiopropyl-L-glutamic acid based N-carboxyanhydride (MTPLG-NCA). Polypeptides bearing sulfonium moieties and different alkyl (i.e., methyl, n-butyl, and propargyl) pendants were prepared via the alkylation of PMTPLG. They are able to show upper critical solution temperature (UCST)-type thermoresponsive properties in methanol or ethanol depending on the alkyl pendants and counter-anions. Polypeptides bearing sulfonium linkages, ammonium pendants and tetrafluoroborate (BF4−) counter-anions (PPLG-MSEA-BF4) were prepared via the copper-mediated [2 + 3] alkyne–azide 1,3-dipolar cycloaddition of polypeptides bearing sulfonium moieties and propargyl pendants, and subsequently ion-exchange reaction. They showed UCST-type thermo and pH dual responsiveness in aqueous solutions. The sharp solution phase separation of PPLG-MSEA-BF4 occurred in a very small pH range (ΔpH = 0.05) when the pH increased from 7.37 to 7.42 as suggested by dynamic light scattering (DLS). The UCST-type phase transition temperature (Tpt) of PPLG-MSEA-BF4 increased by 20 °C as the pH increased from 7.42 to 7.50 as revealed by variable-temperature UV-vis spectroscopy. Increasing polymer or NaBF4 concentrations or decreasing NaCl concentration can further increase the Tpt.


 Please wait while we load your content...
Please wait while we load your content...