Synthesis of fluorinated gradient copolymers via in situ transesterification with fluoroalcohols in tandem living radical polymerization†
Abstract
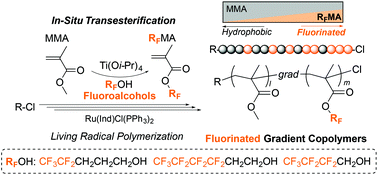
Fluorinated gradient copolymers were synthesized by the tandem catalysis of ruthenium-catalyzed living radical polymerization (LRP) and titanium alkoxide-mediated transesterification of methyl methacrylate (MMA) with fluoroalcohols. Although transesterification using less nucleophilic fluoroalcohols is generally regarded as difficult, we found that MMA was efficiently transesterified with fluoroalcohols (RFOH) into fluorinated methacrylates (RFMA) by Ti(Oi-Pr)4 catalysts (2–8 mol%) in the presence of molecular sieves 4A (MS 4A). The yield of RFMA increased with increasing the alkyl spacer (carbon number) between a hydroxyl group and a fluorinated alkyl segment in fluoroalcohols: propyl (4,4,5,5,5-pentafluoro-1-pentanol: 5FPOH) > ethyl (1H,1H,2H,2H-nonafluoro-1-hexanol: 9FHOH) > methyl (1H,1H-heptafluoro-1-butanol). Tandem polymerization of MMA was conducted with a ruthenium catalyst, a chloride initiator, and Ti(Oi-Pr)4 in toluene/fluoroalcohol mixtures (1/1, v/v) at 80 °C. Typically, in the presence of 4 mol% Ti and MS 4A, transesterification of MMA with 5FPOH or 9FHOH was efficiently synchronized with LRP to produce well-controlled MMA/5FPMA or MMA/9FHMA gradient copolymers in high yield (Conv. >95%, Mw/Mn = 1.2).


 Please wait while we load your content...
Please wait while we load your content...