Systematic variation of thiophene substituents in photochromic spiropyrans†
Abstract
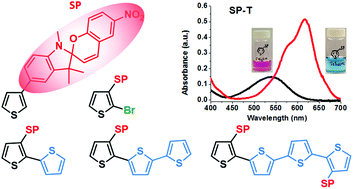
Spiropyrans are notable among different classes of photochromic compounds due to their large structural and electronic transformation upon isomerization. In order to parlay the electronic differences associated with the two isomeric forms into a materials based switch, the spiropyran ultimately requires a covalent attachment through a conjugated pathway. In this work a synthetic method was developed to incorporate spiropyran (SP) into thiophene based materials. A series of compounds with a systematic variation of substituents were synthesized (SP-T, SP-T-Br, SP-T-T, SP-T-T-T and SP-T-T-T-T-SP) and their photochromism in both polar (methanol) and non-polar (toluene) solvents were studied. These compounds showed a systematic variation of photochromic properties.


 Please wait while we load your content...
Please wait while we load your content...