Conformational isomerism in cyclic peptoids and its specification†
Abstract
Most of the structural studies made on the secondary structure of peptoids describe their geometric attributes in terms of the classic Ramachandran plot (based on the local analysis of ω, ψ, χ, φ dihedral angles). However, little intuitive understanding is available from internal coordinates when stereochemistry is involved. In this contribution we list all the conformationally stable cyclic peptoids reported up to the year 2017 and propose a simple method to define their geometric arrangement in terms of planar chirality. Evidence of conformational isomerism (due to the long average time of single bond rotation) and conformational chirality (induced by the absence of roto-reflection axes) in this promising class of synthetic macrocycles is provided by NMR spectroscopy (using Pirkle's alcohol as chiral solvating agent) and careful evaluation of X-ray crystallographic studies. The full understanding of the oligomeric macrocycles’ structural properties and the clear framing of their conformational isomerism in a proper conceptual scheme is fundamental for future application of peptoids in asymmetric synthesis, chiral recognition and supramolecular chemistry.
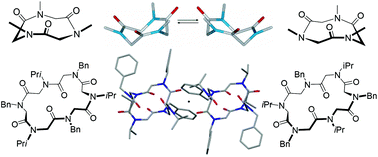
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...