Chain-breaking antioxidant activity of hydroxylated and methoxylated magnolol derivatives: the role of H-bonds†‡
Abstract
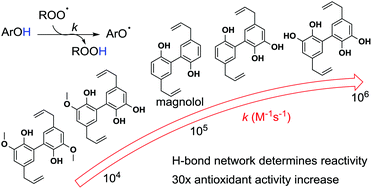
Chemical modification of magnolol, an uncommon dimeric neolignan contained in Magnolia genus trees, provides a unique array of polyphenols having interesting biological activity potentially related to radical scavenging. The chain-breaking antioxidant activity of four new hydroxylated and methoxylated magnolol derivatives was explored by experimental and computational methods. The measurement of the rate constant of the reaction with ROO˙ radicals (kinh) in an apolar solvent showed that the introduction of hydroxyl groups ortho to the phenolic OH in magnolol increased the kinh value, being 2.4 × 105 M−1 s−1 and 3.3 × 105 M−1 s−1 for the mono and the dihydroxy derivatives respectively (kinh of magnolol is 6.1 × 104 M−1 s−1). The di-methoxylated derivative is less reactive than magnolol (kinh = 1.1 × 104 M−1 s−1), while the insertion of both hydroxyl and methoxyl groups showed no effect (6.0 × 104 M−1 s−1). Infrared spectroscopy and theoretical calculations allowed a rationalization of these results and pointed out the crucial role of intramolecular H-bonds. We also show that a correct estimation of the rate constant of the reaction with ROO˙ radicals, by using BDE(OH) calculations, requires that the geometry of the radical is as close as possible to that of the parent phenol.


 Please wait while we load your content...
Please wait while we load your content...