Confined NaAlH4 nanoparticles inside CeO2 hollow nanotubes towards enhanced hydrogen storage†
Abstract
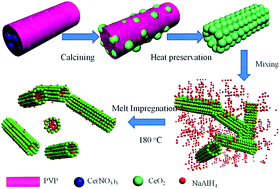
NaAlH4 has been widely regarded as a potential hydrogen storage material due to its favorable thermodynamics and high energy density. The high activation energy barrier and high dehydrogenation temperature, however, significantly hinder its practical application. In this paper, CeO2 hollow nanotubes (HNTs) prepared by a simple electrospinning technique are adopted as functional scaffolds to support NaAlH4 nanoparticles (NPs) towards advanced hydrogen storage performance. The nanoconfined NaAlH4 inside CeO2 HNTs, synthesized via the infiltration of molten NaAlH4 into the CeO2 HNTs under high hydrogen pressure, exhibited significantly improved dehydrogenation properties compared with both bulk and ball-milled CeO2 HNTs-catalyzed NaAlH4. The onset dehydrogenation temperature of the NaAlH4@CeO2 composite was reduced to below 100 °C, with only one main dehydrogenation peak appearing at 130 °C, which is 120 °C and 50 °C lower than for its bulk counterpart and for the ball-milled CeO2 HNTs-catalyzed NaAlH4, respectively. Moreover, ∼5.09 wt% hydrogen could be released within 30 min at 180 °C, while only 1.6 wt% hydrogen was desorbed from the ball-milled NaAlH4 under the same conditions. This significant improvement is mainly attributed to the synergistic effects contributed by the CeO2 HNTs, which could act as not only a structural scaffold to fabricate and confine the NaAlH4 NPs, but also as an effective catalyst to enhance the hydrogen storage performance of NaAlH4.


 Please wait while we load your content...
Please wait while we load your content...