Synthesis and C–H activation reactions of cyclometalated copper(i) complexes with NCN pincer and 1,3,5-triaza-7-phosphaadamantane derivatives: in vitro antimicrobial and cytotoxic activity†
Abstract
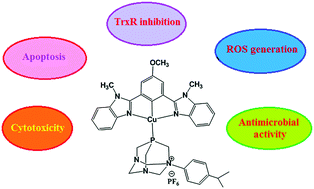
A new class of cyclometalated copper(I) complexes with NCN pincer and N-aryl-1,3,5-triaza-7-phosphaadamantane (PTA-PhR) ligands of formula [Cu(L)(PTA-PhR)](PF6) (R = Me (1), Et (2), iPr (3), and HL = 5-methoxy-1,3-bis(1-methyl-1H-benzo[d]imidazol-2-yl)benzene) have been synthesized by C–H activation and fully characterized. The cytotoxicity of the complexes was evaluated against a panel of several human tumor cell lines. All the complexes showed in vitro antitumor activity comparable to that of the reference metallodrug cisplatin. Tests completed on cisplatin sensitive and resistant cell lines exhibited that against the human ovarian 2008/C13* cell line pair, the resistance factor of the copper complexes was roughly 4–5 lower than that of cisplatin. The thioredoxin reductase activity, reactive oxygen species (ROS) generation, and cellular apoptosis of the complexes have also been studied. Toxicity studies exhibited notable in vitro antimicrobial activity of the copper(I) complexes against Gram-positive and Gram-negative bacterial strains, and they are much more active than furacillinum as a standard drug. The interaction of complexes 1–3 with calf thymus DNA (CT DNA) was investigated using viscosity, fluorescence quenching, and electronic absorption spectroscopy. The DNA cleavage ability of complex 3 has been studied. Overall, the new design of copper(I) complexes provided a useful strategy for the development of bioorganometallic anticancer drugs with multiple modes of action.


 Please wait while we load your content...
Please wait while we load your content...