Preparation of diethylenetriamine-modified magnetic chitosan nanoparticles for adsorption of rare-earth metal ions
Abstract
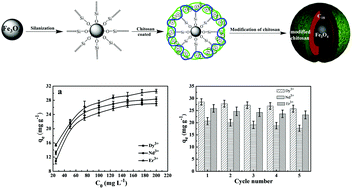
The separation and reutilization of rare-earth metals are flourishing in numerous advanced technologies, yet the development of a sustainable method to conveniently adsorb rare-earth metal ions remains a challenge waiting for a breakthrough. Chitosan, as a natural and biodegradable material, has been frequently studied to synthesize adsorbents for adsorption of rare-earth metals. In this study, a magnetic bio-adsorbent Fe3O4-C18-chitosan-DETA (FCCD) composite was first prepared via a simple “surface deposition-stepwise grafting” method. The results show that the adsorption capacity of Dy3+, Nd3+, and Er3+ to the as-obtained FCCD is about 28.3, 27.1, and 30.6 mg g−1, respectively, at 25 °C and optimal pH (7.0). The kinetics data followed a pseudo-second-order equation, and the Langmuir equation fitted well to the adsorption isotherms. Subsequently, the as-synthesized adsorbent (FCCD) can be successfully regenerated and recycled for 5 cycles using hydrochloric acid as the eluent. This study develops a promising candidate for practical application in the recovery of rare-earth metal ions owing to its magnetic separation, good acid-resistance, and excellent adsorption capacity.

 Please wait while we load your content...
Please wait while we load your content...