Solvent-mediated single-crystal-to-single-crystal transformation from a dimeric to tetrameric copper(i) complex based on a substituted pyridine derived from a Diels–Alder adduct†
Abstract
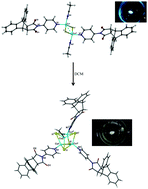
A condensation reaction between the Diels–Alder adduct of anthracene and maleic anhydride and 4-aminopyridine leads to the formation of N-(4-pyridinyl)-9,10-dihydroethanoanthracene-11,12-dicarboximide (L). The reaction of L and CuI in acetonitrile in the presence of saturated NaI facilitates the formation of a dimeric complex, [Cu2I2L2(MeCN)2] (1). By immersing 1 in dichloromethane (DCM), a tetrameric structure, [Cu4I4L3(MeCN)] (2), was obtained, which indicated solvent-mediated single-crystal-to-single-crystal (SCSC) transformation. The observed SCSC transformation promoted higher nuclearity in the formation of a cubane type structure from a dinuclear cluster complex. Herein, the DCM solution assisted the removal of MeCN and L from the crystal of 1, leading to an unprecedented and interesting SCSC transformation. While the physical appearances of both crystals were similar, the SCSC transformation was confirmed by the different emissions of both complexes under a hand-held UV lamp. Compound 1 displayed a fascinating photoluminescence property and was a brightly emissive complex at room temperature, showing an intense blue.


 Please wait while we load your content...
Please wait while we load your content...