Photoinduced oxygen prompted iron–iron oxide catalyzed clock reaction: a mimic of the blue bottle experiment†
Abstract
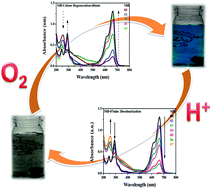
Mixed-valent iron–iron oxide nanoparticles were synthesized by the reduction of Fe2+ to Fe0 followed by aerial oxidation, and were fully characterized using powder XRD, FE-SEM, EDX, and XPS. A clock reaction of organic dyes (methylene blue, nile blue and crystal violet) was performed with iron–iron oxide nanoparticles, where periodic colour changes were observed. The decolourization of the dyes was very fast (within 7–12 min), whereas colour regeneration took a relatively longer time (up to 90 min). Furthermore, this clock reaction was found to be dependent on light and atmospheric oxygen. Light speeds up the generation of electron–hole pairs that are responsible for the reduction of the dyes, whereas oxygen is required for the colour regeneration.


 Please wait while we load your content...
Please wait while we load your content...