n-Capric acid-anchored silanized silica gel: its application to sample clean-up of Th(iv) sorbed as a dinuclear species in quantified H-bonded dimeric metal-trapping cores†
Abstract
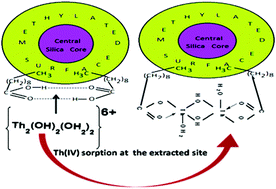
The selective separation, preconcentration and recovery of Th(IV) from aqueous solutions were investigated using reusable (>800 cycles @ 95% recovery) silanized silica gel impregnated with n-capric acid (nCA) (SSG–nCA) via batch column adsorption methods. SSG–nCA is a porous material (pore volume: 2.21 mL g−1) with a large Brunauer–Emmett–Teller (BET) surface area (1820 m2 g−1). Dimethyldichlorosilane (DMDCS) binds unit cells of the SG skeleton (i.e., {Si(OSi)4·xH2O}θ=2.4) via an {SiO2}n=12–O–Si(Me)2–O–{SiO2}n=12 3-D network to produce SSG by an intra-particle silanization reaction. In SSG–nCA, long hydrophobic hydrocarbon chains of nCA, which are anchored to the hydrophobic surface of SSG, generate 121 μmol g−1 H-bonded dimeric metal-trapping cores (HBDMTC), which are oriented towards the hydrophilic mobile phase. It exhibited significant sorption of Th(IV) (breakthrough capacity (BTC): 235 ± 15 μmol g−1; minimum sorption equilibrium time: >12 minutes; high recovery: >95% from a large sample volume of 1000 mL; and a high preconcentration factor (PF): 192). The dimeric aquo-{Th2(OH)2(H2O)2}6+ sorbed species that was identified anchored with an appreciable binding energy (−38.37 eV per mole) at the optimum pH of 5.0 ± 0.4. Together with the existing standard methodology, density functional theory (DFT)-based computations were performed for the characterization of both the extractant and the extracted species. The sorption process was endothermic (+ΔH), entropy-increasing (+ΔS) and spontaneous (−ΔG) in nature. It was found to be effective in the presence of 0.125–0.150 mmol mL−1 Na/K salts of coexisting ions. The sequential separation of Zr(IV), U(VI), Ce(IV), and Th(IV) was achieved by exploiting the differences in the pH used for extraction (Zr(IV) at a pH of 2.5 and U(VI), Ce(IV), and Th(IV) at a pH of 5.0 ± 0.4), followed by their selective elution from the respective extracted portion (Zr(IV): 4 M HNO3, U(VI): 0.6 M CH3COOH, Ce(IV): 1 M CH3COOH, and Th(IV): 0.5 M HNO3).


 Please wait while we load your content...
Please wait while we load your content...