Phosphorus–nitrogen compounds. Part 37. Syntheses and structural characterizations, biological activities of mono and bis(4-fluorobenzyl)spirocyclotetraphosphazenes†
Abstract
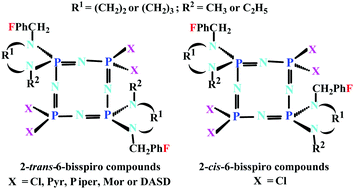
The Cl substitution reactions of octachlorocyclotetraphosphazene, N4P4Cl8, with one equimolar amount of (4-fluorobenzyl)diamines (1–3) affords mono(4-fluorobenzyl)spirocyclotetraphosphazenes (4–6) as minor products. However, the reactions of N4P4Cl8 with two equimolar amounts of (4-fluorobenzyl)diamines (1–3) leads to the formation of mono (4–6), 2-trans-6-bis (7–9, as major products) and 2-cis-6-bis (4-fluorobenzyl)spirocyclotetraphosphazenes (10–12). The 2-cis-6-bis compounds (10 and 12) were separated and purified using column chromatography as minor products, whereas compound 11 could not be isolated. The mono-spiro (4–6) and 2-trans-6-bis-spiro (7–9) cyclotetraphosphazenes were reacted with excess pyrrolidine in THF to afford the fully substituted hexapyrrolidino (4a-6a) and tetrapyrrolidino (7a-9a) products in high yield. Compound 9 was also reacted with piperidine, morpholine and 1,4-dioxa-8-azaspiro[4,5]decane (DASD) to obtain the tetraamino products (9b, 9c and 9d), respectively, due to its very high yield. The elemental analyses, mass spectra (ESI-MS), Fourier transform infrared (FTIR) spectra, and 1H, 13C{1H}, and 31P{1H} NMR data of the cyclotetraphosphazenes were in agreement with the suggested structures. The molecular structures of 7, 9 and 12 were established by X-ray crystallography. The antibacterial activities of the compounds against G(+) and G(−) bacteria and their antifungal activities against yeast strains were scrutinized. The results indicated that 4a and 5a were the most active compounds, especially to yeast strains. In addition, it was found that the most active compound toward DNA was 8. The cytotoxic activities of the cyclotetraphosphazenes against L929 fibroblast and MCF-7 breast cancer cell lines were elucidated. Compound 8a exhibited the most toxic effects against both types of cells.


 Please wait while we load your content...
Please wait while we load your content...