Rh/Ni wet-impregnated Ia3d mesostructured aluminosilicate and r-GO catalysts for hydrodeoxygenation of phenoxybenzene
Abstract
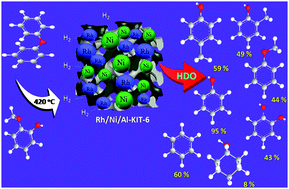
Rh/Ni bimetallic supported bifunctional 3D porous aluminosilicate and Rh/Ni supported reduced graphene oxide (r-GO) catalysts were synthesised and their structural properties evaluated by XRD, BET-surface area, FT-IR, NH3-TPD, H2-TPR, ICP-OES, HRTEM-EDAX and XPS analysis. The catalytic activities of the Rh/Ni/Al-KIT-6 and Rh/Ni–rGO catalysts were investigated by analysis of the vapour phase hydrodeoxygenation (HDO) of diphenyl ether and guaiacol. The Rh/Ni/Al-KIT-6 catalyst exhibited the best catalytic performance for vapour phase HDO of diphenyl ether at 420 °C with a WHSV of 3.6 h−1. In addition, lignin derived guaiacol was also investigated and the desired product of benzene was not achieved for both the Rh/Ni/Al-KIT-6 and Rh/Ni/r-GO catalysts. The Rh/Ni/Al-KIT-6 catalyst demonstrated excellent stability, due to the acidic Al-KIT-6 support which stabilizes the Rh/Ni particles on the surface. However, a higher dispersion of Rh/Ni species was observed on the Rh/Ni/Al-KIT-6 catalyst, as evidenced by HR-TEM imaging, which is mainly due to the large surface area of the Al-KIT-6 support, while the Rh/Ni/r-GO catalyst exhibited larger metal aggregates due to weak Rh/Ni interactions with the reduced graphene oxide (r-GO) support, resulting in a smaller surface area for this catalyst which could restrict the distribution of Rh/Ni particles. Superior catalytic activity towards the C–O bond cleavage of diphenyl ether and guaiacol was observed for the Rh/Ni/Al-KIT-6 catalyst. The superior catalytic activity of this catalyst is mainly due to the highly dispersed small Rh/Ni bimetallic species and their interactions with the mesoporous cubic acidic support via the hydrogen spill over effect, which favours the earlier NiO reduction, and which was confirmed by H2-TPR and HR-TEM. The Rh/Ni/Al-KIT-6 catalyst exhibit excellent catalytic properties, with 60% benzene selectivity observed. Furthermore, the bifunctional behaviour of the Rh/Ni/Al-KIT-6 catalyst, with its unique cubic morphology support in addition to the uniform dispersion of Rh/Ni species, could enhance diphenyl ether conversion and benzene selectivity.


 Please wait while we load your content...
Please wait while we load your content...