Dual antioxidant/pro-oxidant behavior of the tryptophan metabolite 3-hydroxyanthranilic acid: a theoretical investigation of reaction mechanisms and kinetics†
Abstract
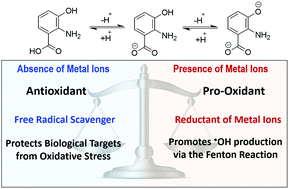
The antioxidant and pro-oxidant behavior of 3-hydroxyanthranilic acid was investigated using density functional theory. In the absence of metal ions 3-hydroxyanthranilic acid acts as an excellent antioxidant by scavenging free radicals. It was found to be an excellent peroxyl radical scavenger in both lipid and aqueous solution, reacting with ˙OOH faster than Trolox. Moreover, the gathered kinetic data support the idea that 3-hydroxyanthranilic acid significantly contributes to the antioxidant activity usually attributed to tryptophan. In contrast, in the presence of metal ions (at physiological pH) it exhibits pro-oxidant behavior. This behavior arises from the Cu(II) reducing ability of the anionic fractions of this compound, which would contribute to producing Cu(I) and consequently promote ˙OH production via the Fenton reaction. Accordingly, the environmental factor identified to be crucial for ruling the dual behavior of 3-hydroxyanthranilic acid is the presence of metal ions. In addition, the pH is also predicted to influence the pro-oxidant effects of this compound.


 Please wait while we load your content...
Please wait while we load your content...