Amino- and chloro-8-hydroxyquinolines and their copper complexes as proteasome inhibitors and antiproliferative agents†
Abstract
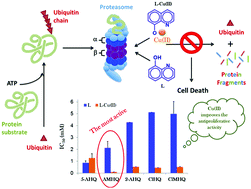
Proliferation and programmed cell death are tightly correlated with the ubiquitin–proteasome system (UPS). Alterations in the UPS may be implicated in pathological conditions such as the proteasome over-activity in cancer cells. Mounting evidence indicates that many types of actively proliferating malignant cells are more sensitive to proteasome inhibition than normal cells, and therefore UPS inhibitors are actively pursued as anticancer agents. The approval of the proteasome inhibitor drug bortezomib for the treatment of myeloma and lymphoma further highlights the need for UPS inhibitors. Recent studies have suggested that clioquinol and 5-amino-8-hydroxyquinoline can inhibit proteasome activity and induce apoptosis in human cancer cells. As for clioquinol, a copper-dependent and -independent mechanism has been proposed to explain the inhibition of the proteasome whereas the activity of 5-amino-8-hydroxyquinoline has not been explored in the presence of copper(II) ions. Herein, we investigated the biological activity of some 8-hydroxyquinolines by using human ovarian (A2780) and lung (A549) cancer cells. The effect of copper(II) on the activity of these compounds was also evaluated. The investigated systems inhibit the chymotrypsin-like activity of the proteasome and induce growth inhibition and apoptosis in a concentration-dependent manner. Copper(II) ions increase the activity of 8-hydroxyquinoline derivatives except in the case of 5-amino-8-hydroxyquinoline. This study suggests the great potential of amino- and chloro-8-hydroxyquinolines as anticancer agents. Furthermore, it clarifies some aspects concerning the activity of 5-amino-8-hydroxyquinoline, which has been previously proposed as a proteasome inhibitor capable of overcoming resistance to bortezomib.


 Please wait while we load your content...
Please wait while we load your content...