Microfluidic modeling of the biophysical microenvironment in tumor cell invasion
Abstract
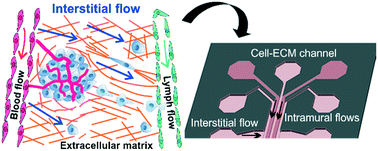
Tumor cell invasion, whether penetrating through the extracellular matrix (ECM) or crossing a vascular endothelium, is a critical step in the cancer metastatic cascade. Along the way from a primary tumor to a distant metastatic site, tumor cells interact actively with the microenvironment either via biomechanical (e. g. ECM stiffness) or biochemical (e.g. secreted cytokines) signals. Increasingly, it is recognized that the tumor microenvironment (TME) is a critical player in tumor cell invasion. A main challenge for the mechanistic understanding of tumor cell–TME interactions comes from the complexity of the TME, which consists of extracellular matrices, fluid flows, cytokine gradients and other cell types. It is difficult to control TME parameters in conventional in vitro experimental designs such as Boyden chambers or in vivo such as in mouse models. Microfluidics has emerged as an enabling tool for exploring the TME parameter space because of its ease of use in recreating a complex and physiologically realistic three dimensional TME with well-defined spatial and temporal control. In this perspective, we will discuss designing principles for modeling the biophysical microenvironment (biological flows and ECM) for tumor cells using microfluidic devices and the potential microfluidic technology holds in recreating a physiologically realistic tumor microenvironment. The focus will be on applications of microfluidic models in tumor cell invasion.


 Please wait while we load your content...
Please wait while we load your content...