Cyclic ureas (DMI, DMPU) as efficient, sustainable ligands in iron-catalyzed C(sp2)–C(sp3) coupling of aryl chlorides and tosylates†
Abstract
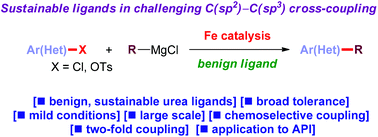
Iron-catalyzed cross-coupling has emerged as a powerful tool for sustainable catalysis. However, by far the most common ligand in iron-catalyzed cross-couplings for preparative and industrial applications is reprotoxic NMP. Herein, we report that cyclic ureas (DMI, DMPU) are efficient and sustainable alternatives to NMP in iron-catalyzed alkylations of aryl chlorides and tosylates with alkyl Grignard reagents. This environmentally benign method accomplishes traditionally challenging C(sp2)–C(sp3) cross-coupling with organometallics possessing β-hydrogens with efficiency matching or superseding NMP. The reaction is compatible with a variety of electrophilic functional handles. Applications to double and site-specific alkylations are described. The potential of this method is highlighted in the key coupling in the synthesis of a dual NK1/serotonin receptor antagonist. Considering the pivotal importance of sustainable iron-catalysis, we believe that the method will find wide application in green chemistry.


 Please wait while we load your content...
Please wait while we load your content...